The following Terms of Reference will shortly be updated to reflect the change in name from RINTAG to ROFG (Research Operational Feasibility Group).
NHSBT is keen to work with its stakeholders to support innovation and research and, when appropriate, help coordinate implementation of those developments and technologies that will lead to improved rates of organ donation or improved organ function and better outcomes for patients in need of solid organ transplants.
The Research, Innovation and Novel Technologies Advisory Group (RINTAG) has developed from the Novel Technologies in Organ Transplantation Working Party, with the aims of providing NHSBT and other stakeholders with an overview of current innovations and supporting the implementation of appropriately approved and funded research, innovations and service development, horizon scanning and working with commissioners and others to ensure the introduction of novel approaches to improve the outcomes of patients undergoing solid organ transplantation, in line with the UK Strategy ‘Taking Organ Transplantation to 2020’.
1. Terms of Reference
1.1. The roles of the Advisory Group include:
1.1.1. Research Strategy Group: RINTAG will take on the remit of NHSBT’s Research Strategy Group in Organ Donation and Transplantation (The generic terms of reference for these groups are shown in the Appendix).
1.1.2. Horizon Scanning: review and advise NHSBT and other stakeholders of current and future technologies involving organ donation, retrieval and transplantation that may impact on service delivery.
1.1.3. Current research, innovation and service development: provide an oversight of current innovations, research and service developments affecting the donation and transplant pathway in order to coordinate the smooth implementation of approved studies, reduce duplication and avoid potential conflicts.
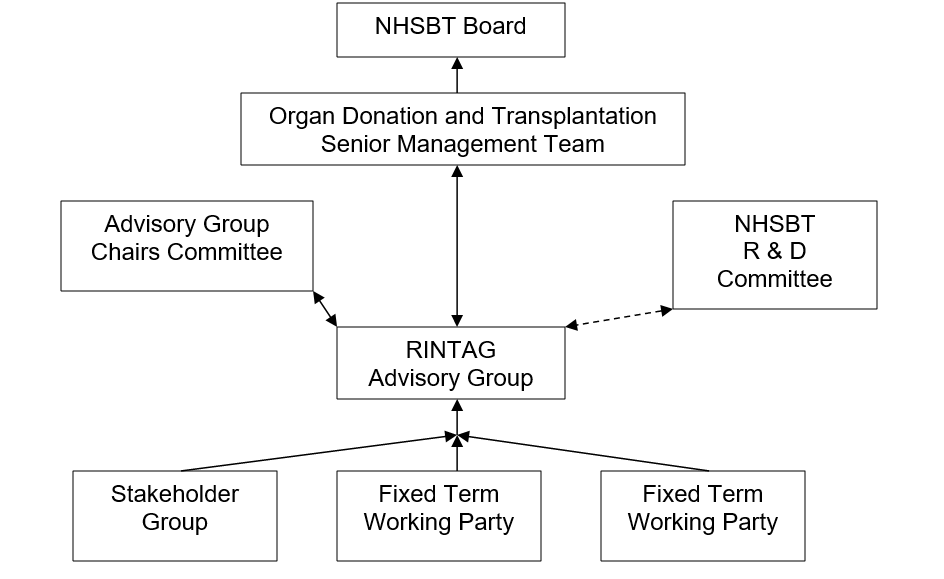
1.1.4. NHSBT supported projects: RINTAG will work with others to ensure timely initiation and implementation of NHSBT projects, review progress reports from such projects, and advise NHSBT’s Research and Development Committee, Advisory Group Chairs Committee or the Senior Management Team of the Organ Donation and Transplantation Directorate (ODT SMT) as appropriate.
1.1.5. Support implementation of proposed research, innovation and service development: RINTAG’s executive group will review protocols of proposed research, innovation and service developments to ensure that, prior to commencement, the appropriate approvals and other governance arrangements are in place, that the protocols are clear and comprehensive and that all relevant interested parties have been consulted and given approval and the integrity of the donation and transplantation process is not jeopardized. It should be noted that NHSBT is not constituted to give advice on research ethics and that permission to approve significant changes, such as uterus or face transplants, will be given by the ODT SMT of NHSBT with advice from RINTAG.
1.1.6. Facilitate research proposals and access to organs and tissue for research purposes:
1.1.6.1. NHSBT is keen to facilitate the retrieval of organs and tissue for research. Requests for organs and tissues are received from many sources including UK clinicians and scientists in hospitals and universities as well as pharmaceutical and other companies. NHSBT is also committed to support the QUOD project.
1.1.6.2. RINTAG will review the current NHSBT policies for access to organs and tissue for research (including cost recovery where appropriate) and make recommendations where appropriate to ensure that NHSBT can provide an effective and efficient service that meets the demands of researchers in a fair, transparent, efficient and equitable manner and fulfill the wishes of the donors.
1.1.6.3. RINTAG will monitor implementation of these policies.
1.1.7. Help NHSBT and relevant stakeholders develop the business cases for the novel technologies and innovations that are determined as beneficial for increasing the number and quality of the donor organs based on the best clinical evidence.
1.1.8. Funding: RINTAG will work to identify funding opportunities to support research, innovation and service development.
1.1.9. RINTAG will also provide advice and support to the Department of Statistics and Clinical Studies as requested, with respect to access for data and statistical support.
1.1.10. For the Organ Donation and Transplantation Directorate, RINTAG will assume the role of NHSBTs Research Strategy Group and make recommendations to the ODT Senior Management Team. This includes the terms of reference;
1.1.10.1. To be the forum in which representatives of research, development, operations and marketing/customer relations for a specific business area of NHSBT meet, in order to ensure that:
- a. Operational requirements which can be solved by either research or product development are considered for inclusion in the rolling R&D programme.
- b. Operational support for R&D projects is considered at the planning stage, costed into research proposals, and reviewed as the project progresses.
- c. Operational teams are fully aware of ongoing research and are therefore able to plan for translation of the research through clinical evaluations into routine products and services.
- d.Operational facilitation of studies requiring specific research consent and HTA license requirements is adequately managed. For such studies, RINTAG will assume the capacity and capability assessment as part of its approval. In addition to RINTAG approval, such studies will also require sign-off by the NHSBT R&D Office.
1.1.10.2. To consider changes to clinical care, scientific developments, the political landscape, and actions of competitors that impact on NHSBT’s product or service provision.
2. Membership and frequency of meetings
2.1. The Chair will be a senior clinician or scientist actively working in the field of organ donation, retrieval or transplantation.
2.1.1. The Advisory Group membership will consist of:
- Chairs (or deputies) of solid organ advisory groups (Heart and Lung to have separate representation), National Retrieval Group and National Organ Donation Committee
- Director of Organ Donation and Transplantation
- Director of The Quality in Organ Donation (QUOD)*
- NHSBT Assistant Director – Research & Development
- NHSBT Statistics and Clinical Studies
- ODT Research Manager
- Head of Transplant Development, ODT
- Associate Medical Director, ODT
- National Quality Manager, ODT
- Specialist Nurse Research and Service Delivery
- Assistant Director for Organ
- Donation and Nursing, ODT
- Assistant Director for Commissioning, ODT
- NHSBT Finance
- National Clinical Lead – Governance, ODT
- Representative from the Cambridge/Newcastle NIHR BTRU*
- Representative from the British Transplantation Society (BTS)
- Lay members
* Non-voting members
2.2. Executive group
2.2.1. The aim of the executive group is stipulated in section 1.1.5 above, including the regular assessment of the allocation ranking exercise, as required by the new allocation policy for research organs.
2.2.2. The executive group membership will consist of:
- Chair
- Associate Medical Director, ODT
- Chair, Kidney Advisory Group
- Chair, Pancreas Advisory Group
- Chair, Bowel Advisory Group
- Chair, Liver Advisory Group
- Chair, Cardiothoracic Advisory Group
- Chair, National Organ Donation Committee
- Chair, National Retrieval Group
- Assistant Director – Research & Development, NHSBT
- Assistant Director for Commissioning, ODT
- Assistant Director for Organ
- Donation and Nursing, ODT
- National Quality Manager, NHSBT
2.3. Frequency of meetings – Wider Group
2.3.1. RINTAG will meet for a face-to-face meeting every six months.
2.3.2. The Group will be consulted via email in the interim period between meetings for any relevant correspondence and cases requiring more urgent responses.
2.4. Frequency of meetings – Executive Group
2.4.1. Email consultation with RINTAG’s Executive Group takes place every quarter for the purpose of reviewing new proposals and assessing the allocation ranking exercise.
2.4.2. Working by teleconferencing, videoconferencing and email is encouraged for the frequency of any interim meetings as required.
2.5. Working parties
The Advisory Group may commission fixed-term short-life working parties to address specific issues in a timely manner. There will be no more than two such working parties at any one time.
2.6. Annual Stakeholder meeting
2.6.1. The remit of the Advisory Group is wide, so it is essential that there is effective communication with the broad spectrum of stakeholders. RINTAG will therefore host an annual Stakeholders’ meeting. Stakeholders will include:
2.6.2. NHSBT representatives
- Director ODT
- NHSBT Medical and Research Director
- Assistant Director, Education and Excellence
- Assistant Director, Transplant Support Services
- ODT National Lead – Clinical Governance
- ODT National Lead – Medical Informatics
- ODT National Lead – Organ Donation
- ODT National Lead – Organ Utilisation
- ODT National Lead – Organ Retrieval
- Associate Director of Statistics and Clinical Studies
2.6.3. Commissioning teams
- Representatives from UK commissioning groups
- Representatives from the National Departments of Health
- Funding bodies
2.6.4. Transplant Health Professionals
- Representative from theatre practitioners (scrub nurses and perfusion practitioners)
- Representative from SNODs
2.6.5. Patient representatives
- Patient representatives, including but not limited to; Cystic Fibrosis Trust, National Kidney Federation; Live Life Give Life; Liver Research Foundation; British Liver Transplant Group; British Heart Foundation
- Donor Family representative
- Lay member
- Representatives from relevant professional bodies, societies and Colleges
3. Support
3.1. Administrative and financial support will be provided by ODT Clinical & Support Services whilst statistical support will be provided by NHSBT Statistics & Clinical Studies to help deliver the aims of the group.
3.2. An annual budget will be set by ODT, in discussion with the Associate Medical Director for Organ Donation and Transplantation and managed by the AMD ODT.
4. Appraisal
4.1. The Chair will meet the Associate Medical Director for Organ Donation and Transplantation every year for a formal review of progress made, to agree targets for future work and a work plan.
5. Minutes
5.1. The notes of each meeting will be taken by the NHSBT (ODT) Secretariat within Clinical & Support Services.
5.2. The approved minutes of meetings of RINTAG and it’s associated working groups will be published on the ODT web-site, together with any papers that are suitable to be placed in the public domain. Any papers that hold sensitive or patient identifiable data etc will be withheld.
5.3. Minutes will be circulated electronically.
6. Reporting Structures
6.1. RINTAG will report formally to ODT SMT
6.2. For items related to the Research Strategy Group, RINTAG will maintain links with the NHSBT R&D Committee.

Terms of reference of NHSBT Research Strategy Groups
Each of the clinical Directorates within NHS Blood and Transplant has has a Research Strategy Group, the Terms of Reference for which are provided below. For the Organ Donation and Transplantation Directorate, RINTAG will assume this role and make recommendations to the ODT Senior Management Team.
1. To be the forum in which representatives of research, development,
operations and marketing/customer relations for a specific business area of NHSBT meet, in order to ensure that:
- a. Operational requirements which can be solved by either research or product development are considered for inclusion in the rolling R&D programme.
- b. Operational support for R&D projects is considered at the planning stage, costed into research proposals, and reviewed as the project progresses.
- c. Operational teams are fully aware of ongoing research and are therefore able to plan for translation of the research through clinical evaluations into routine products and services.
- d. Operational facilitation of studies requiring specific research consent and HTA license requirements is adequately managed. For such studies, RINTAG will assume the capacity and capability assessment as part of its approval. In addition to RINTAG approval, such studies will also require sign-off by the NHSBT R&D Office.
2. To consider changes to clinical care, scientific developments, the political landscape, and actions of competitors that impact on NHSBT’s product or service provision.


